📘 قراءة كتاب Epsiprantel أونلاين


Epsiprantel من كتب طب بيطرى
Evidence Type
A
Good evidence to support a recommendation for use
B
Moderate evidence to support a recommendation for use
1
Species-specific evidence from at least one large randomized and
controlled trial (RCT) or multiple small RCTs
C
Insufficient evidence to support a recommendation for use
D
Moderate evidence to support a recommendation against use
E
Good evidence to support a recommendation against use
2
Species-specific evidence from a small RCT, disease models,
large case studies, pharmacokinetic studies using surrogate
endpoints, or evidence from well-designed trials in a different
species that is considered appropriate for comparison
3
Dramatic results from either well-designed, species-specific trials
without controls, controlled trials without randomization, or small
case studies
4
Pharmacokinetic studies without surrogate endpoints or well
designed pharmacodynamic studies in healthy animals
5
In vitro
studies
6
Opinions of respected authorities on the basis of clinical
experience or reports of expert committees
© 2008 The United States Pharmacopeial Convention
All rights reserved
EPSIPRANTEL Veterinary—Oral-Local
A commonly used brand name for a veterinary-labeled
product is
Cestex
.
Note: For a listing of dosage forms and brand names by
country availability, see the
Dosage Forms
section(s).
Category:
Anthelmintic.
Indications
Note: The text between
EL
US
and
EL
describes uses that
are not included in U.S. product labeling. Text
between
EL
CAN
and
EL
describes uses that are not
included in Canadian product labeling.
The
EL
US
or
EL
CAN
designation may signify a lack
of product availability in the country indicated.
See the
Dosage Forms
section of this monograph
to confirm availability.
Cats
and
dogs
Accepted
Cestode, gastrointestinal, infection (treatment)—
Epsiprantel tablets are indicated in the treatment of
tapeworms,
Dipylidium caninum
in cats and dogs,
Taenia taeniaeformis
in cats, and
Taenia pisiformis
in dogs.
{R-1}
Potentially effective
Cestode, gastrointestinal, infection (treatment)—
Cats
and
dogs:
EL
US,CAN
There is evidence to suggest that
epsiprantel is effective in the treatment of
Echinococcus granulosus
and
Echinococcus
multilocularis;
however, there are insufficient data
to recommend a dosage that can be relied upon to
clear the infection in all animals treated (Evidence
rating: A-1).
{R-9-11}
EL
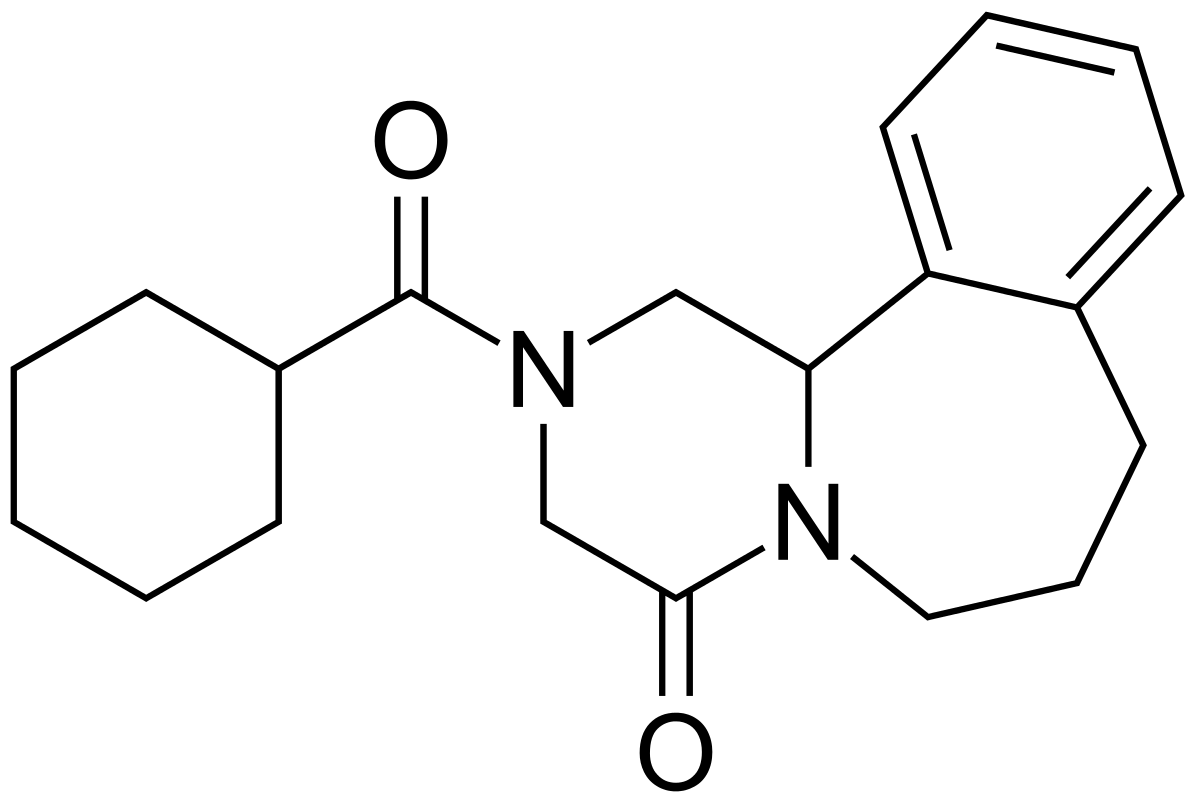
Chemistry
Chemical group:
Pyrazino benzazepine.
{R-5}
Chemical name:
(
±
)-2-(Cyclohexylcarbonyl)-
2,3,6,7,8,12b-hexahydropyrazino[2, 1-
a
][2]benzazepin-4(1
H
)-one.
{R-4}
Molecular formula:
C
20
H
26
N
2
0
2
.
{R-4}
Molecular weight:
326.43.
{R-1; 4}
Description:
A stable, white solid.
{R-1}
Solubility:
Sparingly soluble in water.
{R-1}
Pharmacology/Pharmacokinetics
Mechanism of action/Effect:
The mechanism of
action of epsiprantel appears to be similar to that of
praziquantel, a drug that disrupts the regulation of
calcium and other cations. Tetanic muscle
contraction and paralysis occurs in the parasite, and
the tegument becomes vacuolized.
{R-8; 10}
Absorption:
Minimal absorption occurs in cats and
dogs after oral administration.
{R-1; 5}
Biotransformation:
There is no evidence that
epsiprantel is metabolized.
{R-8}
Concentrations:
Peak plasma concentration—
Cats:
In 83% of cats in one study, the plasma
concentration of epsiprantel was below the level
of detection in all samples taken after an oral
dose of 5.5 mg per kg of body weight (mg/kg).
{R-
8}
When plasma epsiprantel could be measured,
the peak concentration was 0.21 mcg/mL at 30
minutes after administration of the dose.
{R-8}
Dogs:
0.13 mcg/mL (range, <0.5–0.36) at 1 hour
after an oral dose of 5.5 mg/kg.
{R-8}
Elimination:
Cats
and
dogs—
Because only trace
amounts are absorbed, epsiprantel is predominantly
eliminated in the feces.
{R-8}
Less than 0.1% of the
dose is eliminated in the urine in dogs.
{R-8}
Precautions to Consider
Reproduction/Pregnancy
There is no information on the safety of administering
حجم الكتاب عند التحميل : 61.8 كيلوبايت .
نوع الكتاب : pdf.
عداد القراءة:
اذا اعجبك الكتاب فضلاً اضغط على أعجبني و يمكنك تحميله من هنا:

شكرًا لمساهمتكم
شكراً لمساهمتكم معنا في الإرتقاء بمستوى المكتبة ، يمكنكم االتبليغ عن اخطاء او سوء اختيار للكتب وتصنيفها ومحتواها ، أو كتاب يُمنع نشره ، او محمي بحقوق طبع ونشر ، فضلاً قم بالتبليغ عن الكتاب المُخالف:
 قبل تحميل الكتاب ..
قبل تحميل الكتاب ..
يجب ان يتوفر لديكم برنامج تشغيل وقراءة ملفات pdf
يمكن تحميلة من هنا 'http://get.adobe.com/reader/'

 منصّة المكتبة
منصّة المكتبة